Latest Version
Version
1.2
1.2
Update
December 04, 2024
December 04, 2024
Developer
Voladd
Voladd
Categories
Education
Education
Platforms
Android
Android
Downloads
0
0
License
Free
Free
Package Name
com.vlvolad.hydrogenatom
com.vlvolad.hydrogenatom
Report
Report a Problem
Report a Problem
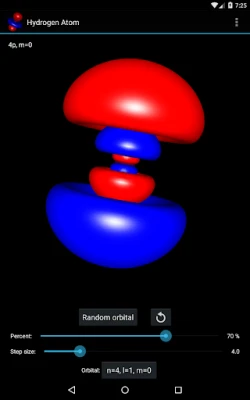
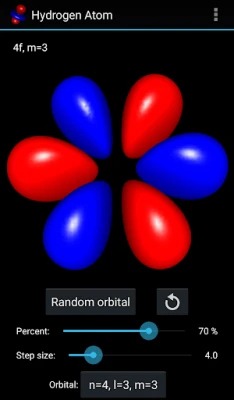
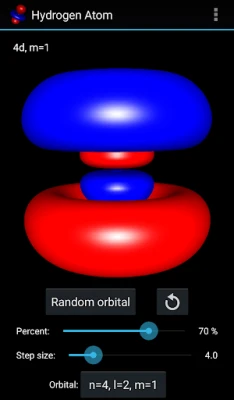
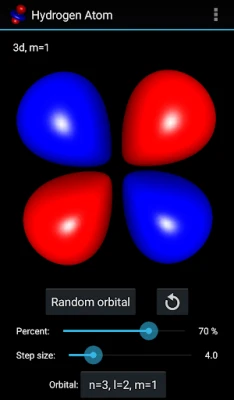
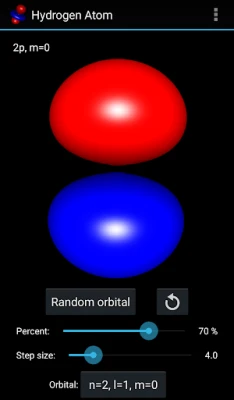
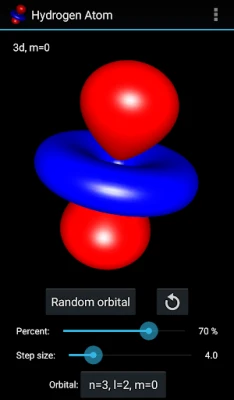
More About Hydrogen Atom Orbitals
The electron orbitals of the hydrogen atom describe the wave-like behavior of an electron in atom and they determine the probability to find it in a particular space region. Mathematically the orbitals are determined by the hydrogen atom wave functions of its energy eigenstates and the wave functions themselves are determined as the solution to the quantum mechanical Schroedinger equation. This app visualizes the electron orbitals of the hydrogen atom...
Rate the App
Add Comment & Review
User Reviews
Based on 0 reviews
No reviews added yet.
Comments will not be approved to be posted if they are SPAM, abusive, off-topic, use profanity, contain a personal attack, or promote hate of any kind.
More »










Popular Apps

Honor of KingsLevel Infinite

Addictive AccountingOne Thumb Learning LLC

Family LifeSupersonic Studios LTD

TSMELECTRONIC ARTS

BitLife - Life SimulatorCandywriter, LLC

Environmental EcologySoftecks

2248 - Number Games 2048Inspired Square FZE

Accounting Quiz GameAccounting Play by John Gillingham CPA

Debit and Credit - AccountingAccounting Play by John Gillingham CPA
Hydrogen Atom OrbitalsVoladd
More »










Editor's Choice

Cashman Casino Slots GamesProduct Madness

Quick Hit Casino Slots GamesSciPlay

Club Vegas Slots Casino GamesBagelcode: Social Casino & Slot Machine Games

TimeKeeper Time and AttendanceArtificial Development
Energy Level TrackerRaven Code

ContactsGoogle LLC

ContactsSWEG ENTERPRISE

SimCity BuildItELECTRONIC ARTS

Pocket City 2Codebrew Games

Titan for Titan mail accountsRiva